
TD-PCR (and its sister technique, stepdown PCR (SD-PCR) see below) has no inherent limitations that are not encountered with most PCR methods, as it simply represents an empirical approach to favoring the most specific primer-template interactions. TD-PCR deals specifically with the limitations inherent in calculations and largely alleviates the need for optimization of reactions and redesigning of primers (6,7). An empirical solution to this problem and a far simpler method to employ is TD-PCR6, which was developed to abrogate mispriming and the production of nonspecific products. However, although these elements can be successful to greater or lesser degrees, their efficacy is largely dependent on trail and error, as what proves effective for one primer set may have little, or even a detrimental, outcome on another primer pair reaction.
The generation of multiple products is often caused by poor melting temperature () estimations and improper annealing conditions, in response to which many different methods have been suggested to increase the specificity of the reaction, ranging from the addition of additives to the reaction mix (e.g., DMSO, formamide, betaine and others), variations in salt concentration and altering the annealing temperature, to name a few (4,5). However, the widespread use of PCR as a diagnostic and preparative technique belies the frustrations that many users have experienced, particularly with regard to the consumption of time and resources in refining and troubleshooting the procedure when spurious products are generated. Since the first papers on PCR were published (1-3) it has become one of the most standard and basic tools in the biological and medical sciences, and advances in engineering and science have increased its scope and usability. The procedure takes between 90 and 120 min, depending on the template length. TD-PCR is particularly useful for templates that are difficult to amplify but can also be standardly used to enhance specificity and product formation.

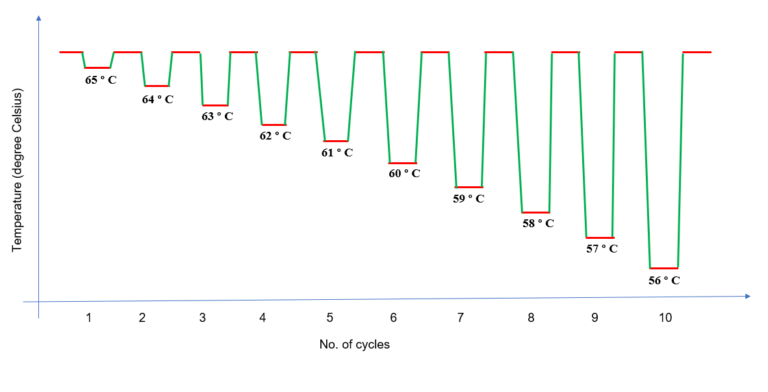
TD-PCR has found wide applicability in standard PCR protocols, including reverse transcriptase-dependent PCR, as well as in the generation of cDNA libraries and single nucleotide polymorphism screening. Any difference in between correct and incorrect annealing will produce an exponential advantage of twofold per cycle. TD-PCR employs an initial annealing temperature above the projected melting temperature () of the primers being used, then progressively transitions to a lower, more permissive annealing temperature over the course of successive cycles. Touchdown (TD) PCR offers a simple and rapid means to optimize PCRs, increasing specificity, sensitivity and yield, without the need for lengthy optimizations and/or the redesigning of primers.


 0 kommentar(er)
0 kommentar(er)
